While the COVID-19 pandemic continues to affect the entire world, more has been learned about the variants, virulence, and transmission of this virus since our last update. Guidance in this area comes from the Centers for Disease Control (CDC), World Health Organization (WHO), American Heart Association (AHA) and the International Liaison Committee on Resuscitation (ILCOR), and the Occupational Safety and Health Administration (OSHA). Ellis & Associates continues to monitor the information provided by these sources so that we may support our clients and the industry at large.
Ellis & Associates continues to monitor the information provided by these sources so that we may support our clients and the industry at large.
What follows represents our efforts to present best practices based on evolving information. This update addresses the two items listed below:
Training Safety Guidelines for Lifeguard and S&H Classes and In-service
Any need for additional precautions should consider local AHJ requirements, training facility restrictions and employer related guidance.
Personal Protective Equipment (PPE) and Hygiene During Class
Every student in a Lifeguard or HCP BLS class should be provided with the following during in-person training:
- Resuscitation mask
- Access to medical exam gloves
- Access to hand sanitizer and/or soap and running water
If a facility is requiring additional PPE to meet local requirements or organization policy, these should be provided during class as appropriate. PPE training should be conducted during initial training and reinforced through pre-service and in-service activities.
Students in Safety & Health classes that do not involve HCP BLS should be given:
- Access to medical exam gloves (First Aid classes)
- Access to hand sanitizer and/or soap and running water
Students in all classes should be allowed to frequently wash their hands with soap and running water and/or be given access to hand sanitizer. Instructors and students should wash their hands/use hand sanitizer at a minimum, during these points in the class:
- The start and end of class.
- When entering or exiting the classroom or pool deck.
- Following an activity that may involve touching equipment, another student, or the ground.
Medical Exam Gloves should be worn whenever the following actions are taking place:
- When practicing skills using a manikin (don during scene safety check).
- When directly working with or touching another student (classroom and pool deck) for BLS or First Aid activities, drills, and demonstrations.
Any need for additional precautions should consider local AHJ requirements, training facility restrictions and employer related guidance where applicable. The resources and precautions provided in the Pectora Library/COVID-19 Folder can be implemented, as needed.
Equipment Cleaning and Disinfection
Training equipment should be cleaned and disinfected before/after class and any equipment that is reused during class should be cleaned and disinfected between uses. Discard items that should not be reused and resupply as needed. The general rule to follow is that all equipment handled by different students should be cleaned and disinfected BEFORE the next student or student group touches the equipment.
Recommended Cleaning and Disinfection Timing:
- Before class – Clean and disinfect any equipment that will be handled by students.
- During class – Provide an appropriate means for students to disinfect equipment they are using before passing the equipment to another student to use or returning it to a bag or other storage.
- Class conclusion – Clean and disinfect any equipment used during class and properly store.
NOTE: Most commercially available disinfectants will have a statement of effectiveness on the label. Suppliers will be able to identify which of their products is effective and appropriate for a particular cleaning or disinfecting purpose.
Cohort System
If the class is taking place in an area of high or substantial community transmission, or the facility requires further precautions, a cohort system can be implemented to accommodate the performance of certain skill competency objectives. Cohorts are small groups of students that will perform all close contact activities only with one another for the duration of the course. Refer to the Pectora Library/COVID-19 Folder/EA Lifeguard Training During COVID-19 Graphics 2022.
Basic Life Support and First Aid Activities
Disinfected manikins should be used when practicing or demonstrating layperson or HCP BLS skills. For layperson CPR, students should be taught rescue breathing, CPR with rescue breathing element, and compression only CPR (focusing on compression only CPR). Surgical masks should be placed over the guest’ during First Aid activities.
In-water Unresponsive Guest Response
Following placement on the rescue tube, a jaw thrust should be applied and maintained, allowing for a quick check for spontaneous breathing, while rapidly moving to the extrication point. At this time, lifeguard response to an in-water, unresponsive guest should continue to omit in-water rescue breathing. Following extrication, the guest in distress should be exchanged for a sanitized manikin and the primary lifeguard (completed the in-water rescue) should put on gloves (and any other PPE being used) before providing on-deck care as needed.
Emergency Care Safety Guidelines
Personal Protective Equipment (PPE) and Hygiene
OSHA has specifically placed the burden on employers to determine the level of risk their employees are subject to while at work, as it relates to COVID-19. State level occupational safety departments may have specific requirements that must be followed. The following recommendations for individual and team PPE and hygiene should be considered the minimum. Additional PPE needed to meet AHJ requirements will be determined by the facility.
Lifeguard/Supervisor Hip Packs:
At a minimum, lifeguard and supervisor hip packs should contain medical exam gloves and a resuscitation mask. A B/V filter may also be placed into hip packs provided the filter remains in its original packaging. Filters need to remain dry to be effective when used. First Aid supplies, hand sanitizer and surgical masks may also be considered for inclusion.
Lifeguards and supervisors should inspect their hip packs to confirm the rescue readiness of each item before going on duty. Hip packs collected at the end of the shift should be cleaned/sanitized before being used the following day.
Trauma Bags
Assembled trauma bags should be checked at the beginning of operating day to confirm all equipment and components are in rescue ready condition.
Responders need to be familiar with the location of each item in the bag; a labeling system is encouraged. It is also recommended that practice trauma bags be set up in the same manner as the live response trauma bag. If multiple team response bags are utilized at a facility, each should be set-up in the same manner to aid in this process.
Recommended Team Response/Trauma Bags Contents:
- Medical exam gloves
- Face masks (for guests)
- Spare B/V filter
- Hand sanitizer
- Disinfecting wipes
- Adult BVM
- Child BVM
- Infant BVM
- Resuscitation mask
- Supplemental oxygen
- Adult NRB
- Pediatric NRB
- Oxygen tubing
- Manual suction
- Pulse oximeter
- AED (including ancillary items)
- Naloxone (if appropriate)
- Epinephrine autoinjector (if appropriate)
- Other PPE in use for responders
- Protective eyewear
Guidelines for Specific Care
The following procedures reflect current ILCOR Interim Guidelines from the 2021 update and related information.
General First Aid Care:
- The first responder puts on PPE
- If the guest is not already wearing a mask, provide one.
- Provide the appropriate care based on signs and symptoms.
- Contact EMS for severe injuries and illnesses or if COVID-19 is suspected.
- Properly clean and disinfect the scene and repeat use equipment.
- Properly dispose of any single use supply or equipment, including worn PPE.
- Wash hands with soap and running water and/or use hand sanitizer.
Supplemental Oxygen Administration:
- The first responder puts on PPE.
- Have the guest sit in a comfortable position as a non-rebreather mask is available for use.
- Place the non-rebreather mask on the guest then place a surgical mask over it.
- Contact EMS.
- Monitor with a pulse oximeter, discontinuing oxygen when the guest is within normal parameters.
- If the non-rebreather mask is removed, have the guest put on the mask that was covering it.
- Transfer care to EMS when they arrive.
- Properly clean and disinfect the scene and repeat use equipment.
- Properly dispose of any single use supply or equipment, including worn PPE.
- Wash hands with soap and running water and/or use hand sanitizer.
Foreign Body Airway Obstruction on a Responsive Guest (over 1 year of age):
- While approaching the scene, the first responder puts on PPE.
- If the guest cannot cough or speak, get into position to perform the Heimlich, and call for help.
- Perform the Heimlich until the object is dislodged or becomes unresponsive.
- If the guest becomes unresponsive, carefully position the guest onto a firm, flat surface.
- Perform 30 chest compressions, then look into the mouth to see if the object is in the airway.
- If an object is seen, remove it.
- The first responder assembles a resuscitation mask and a sterile B/V filter. **
- Carefully create a tight seal and open airway and deliver two breaths looking for chest rise, then resume 30 chest compressions.
- Continue in this manner until the guest resumes breathing on their own or help arrives and takes over care.
** NOTE: If a resuscitation mask & BV filter is not immediately available, continue with 30 chest compressions and re-checking the airway (and repeat), until object is dislodged/breathing is restored, or until the mask and filter is available.
Foreign Body Airway Obstruction on a Responsive Guest (under 1 year of age):
- While approaching the scene, the first responder puts on PPE.
- Perform 5 back blows and 5 chest thrusts until the infant dislodges the object or becomes unresponsive.
- If the infant becomes unresponsive, carefully position the guest onto a firm, flat surface.
- Perform 30 chest compressions, then look into the mouth to see if the object is in the airway.
- If an object is seen, pluck the object out, if seen.
- The first responder assembles a resuscitation mask and a sterile B/V filter. **
- Carefully create a tight seal and open airway (neutral position) and deliver two breaths looking for chest rise, then resume 30 chest compressions.
- Continue in this manner until the guest resumes breathing on their own or help arrives and takes over care.
** NOTE: If a resuscitation mask & BV filter is not immediately available, continue with 30 chest compressions and re-checking the airway (and repeat), until object is dislodged/breathing is restored, or until the mask and filter is available.
Single Rescuer BLS:
- While approaching the scene, the first responder puts on PPE.
- If the scene is safe, check for responsiveness, if not responsive, call for help (if not already done).
- Place the guest face up on a firm, hard surface and assess pulse and breathing for up to 10 seconds.
- Assess breathing by looking for chest movement while checking for the pulse
- If the guest has a pulse but is not breathing and a resuscitation mask and sterile B/V filter is available, deliver rescue breathing for 2 minutes. Reassess pulse/breathing after 2 minutes **
- If the guest does not have a pulse or breathing and a resuscitation mask and sterile B/V filter is available, begin 30 chest compressions followed by 2 breaths (continue until AED arrives or guest exhibits signs of life).
** NOTE: If the first responder does not have access to a resuscitation mask with a B/V Filter, compression only CPR should be initiated until the equipment becomes available and/or additional responders take over care.
- Upon arrival of the AED, secondary responder(s) - if properly trained - should put on all PPE used at the facility for BLS care, while the first responder continues care (including AED use). When ready, secondary responder(s) should enter the scene and take over care while the initial responder puts on additional PPE. Responders should subsequently provide the maximum level of care appropriate for the number of responders available.
- When finished, responders should properly dispose of used equipment, including any PPE and sanitize, wash, and rinse their exposed skin.
- Properly dispose of any single use supply or equipment, including worn PPE, and clean/disinfect the scene and any reusable equipment.
- Wash hands and any exposed skill with soap and running water and/or use hand sanitizer.
In-water Unresponsive Guest – In-water and On-deck Team BLS Care:
- “Primary” Lifeguard recognizes the unresponsive guest, activates the EAP and safely enters the water.
- Additional responding lifeguards follow EAP, including moving equipment to the extrication point.
- Primary performs an appropriate rescue and places the guest face up on the rescue tube.
- Primary uses a jaw thrust to maintain an open airway while quickly assessing for spontaneous breathing.
- Primary continues with the guest to the extrication point.
- At least one responding lifeguard (“Secondary”) wearing a mask and gloves should be ready at the extrication point to assist the primary in rapid extrication. Other responders should be putting on the PPE used at the facility.
- Primary and Secondary safely extricate the guest on to the deck.
- Secondary assesses pulse and breathing for up to 10 seconds.
- Assess breathing by looking for chest movement while checking for the pulse
- Primary puts on PPE, assembles a resuscitation mask with B/V filter, if available
- If no pulse or breathing are found, one rescuer begins 30 chest compressions and calls for an AED while the other rescuer prepares to deliver ventilations.
- Responding lifeguards provide the AED to the scene.
- If a pulse is found, but no breathing, one rescuer should perform rescue breathing.
- If no pulse or breathing are found, one rescuer begins 30 chest compressions and calls for an AED while the other rescuer prepares to deliver ventilations.
NOTE: If the first responder does not have access to a resuscitation mask with a B/V Filter, compression only CPR should be initiated until additional responders take over care.
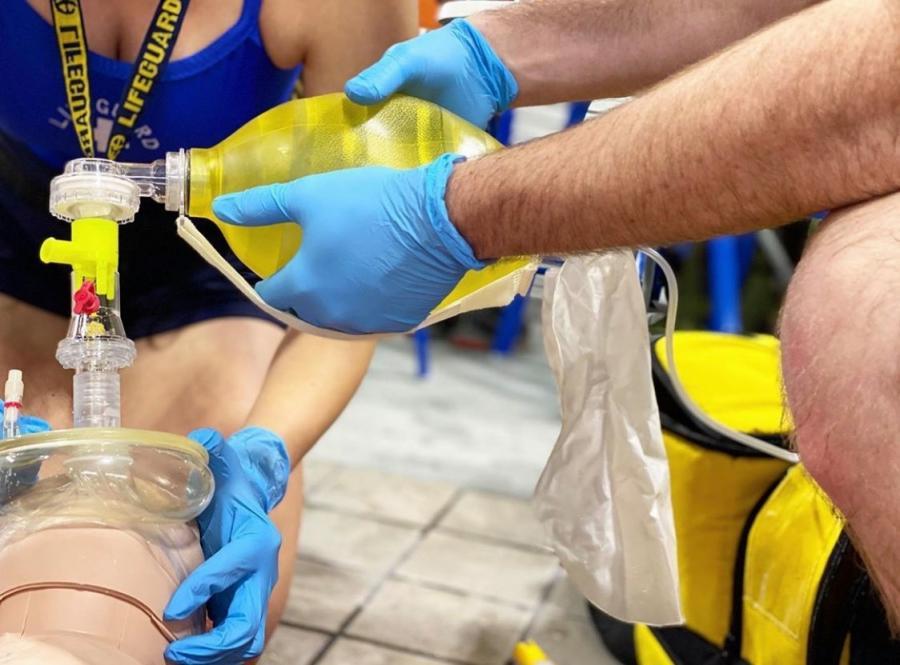
Using a Bag Valve Mask during Care:
- Select the most appropriately sized BVM to match the guest from the trauma bag.
- Remove the BVM and connect it to supplemental oxygen.
- Remove the B/V filter and connect it to the resuscitation mask (the filter is always placed before the one-way-valve, which is an exhaust) then connect the resuscitation mask to the BVM.
- Turn on the flow of oxygen and allow the reservoir bag to inflate.
- The responder managing the airway carefully places the BVM mask onto the guest’s face to create a tight seal while also maintaining an open airway.
- The responder holding the bag of the BVM delivers ventilations by squeezing the bag for 1 second, looking for chest rise and continuing ventilations in time with CPR or rescue breathing care.
NOTE: The B/V filter can only protect if the BVM/resuscitation mask is properly sealed at all times, including during chest compressions. The BVM/resuscitation mask should only be removed to clear the airway if needed.

